PRODUCTO
Acarbose
| Chemical Name | : Acarbose |
| Category | : Hypoglycemic |
| Specification | : EP |
| HS Code | : 2932.9990.99 |
- Teléfono: +86-532-83876123
- Fax: +86-532-83876157
- Email: dennis@qingmeibio.com
- Skype: dennis10221
LAS CARACTERíSTICAS QUíMICAS
| Structure Formula | : 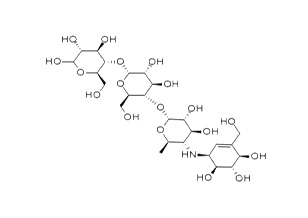 |
| CAS Number | : 56180-94-0 |
| Molecular Formula | : C25H43NO18 |
| Usage | : Acarbose is a novel oral hypoglycemic agents. In the intestine of competitive inhibition of glycoside hydrolase. Reduce the polysaccharide and sucrose into glucose, the sugar uptake corresponding to slow down, so it can be with the postprandial blood glucose lowering effect. Generally used alone, or with other oral hypoglycemic agents or insulin therapy. With the food and beverage, treatment of insulin dependent and non dependent diabetes mellitus. Acarbose for carbohydrates in the small intestine and is absorbed slow decomposition, prolong the retention time, the intestinal bacteria glycolysis and gas production increased, so it can cause abdominal distension, abdominal pain and diarrhea |
LOS PARÄMETROS TËCNICOS
| Items | Standard |
|---|---|
| Appearance | White or yellow, amorphous powder, hygroscopic. Very solution in water, soluble in methanol, practically insoluble in methylene chloride |
| Identification | As per EP7.0 |
| Accordant with IR spectrum obtained | |
| with reference standard | |
| Absorbance | Max.0.15 at 425nm for solution S |
| Assay(on anhydrousbasis) | 95.0%-102.0% |
| Water | ≤4.0% |
| Specific optical rotation | +168°~+183° |
| Sulfated ash | ≤0.2% |
| PH | 5.5~7.5 |
| Heavy metals | ≤20ppm |
| Impurity A | ≤0.6% |
| Impurity B | ≤0.5% |
| Impurity C | ≤1.5% |
| Impurity D | ≤1.0% |
| Impurity E | ≤0.2% |
| Impurity F | ≤0.3% |
| Impurity G | ≤0.3% |
| Impurity H | ≤0.2% |
| Any other impurity | Each impurities ≤0.2% |
| Total impurities | ≤3.0% |
| Bulk density | 0.50-0.65g/ml |
| Tapped density | 0.70-0.90g/ml |
| Total plate count | ≤1000vfu/g |
| Mould | ≤100vfu/g |
LOS DOCUMENTOS EN APOYO
The available documents are:
Chinese GMP
CEP
DMF/CTD format
The available registered countries are:
Algeria
Spain
Australia
Chinese GMP
CEP
DMF/CTD format
The available registered countries are:
Algeria
Spain
Australia